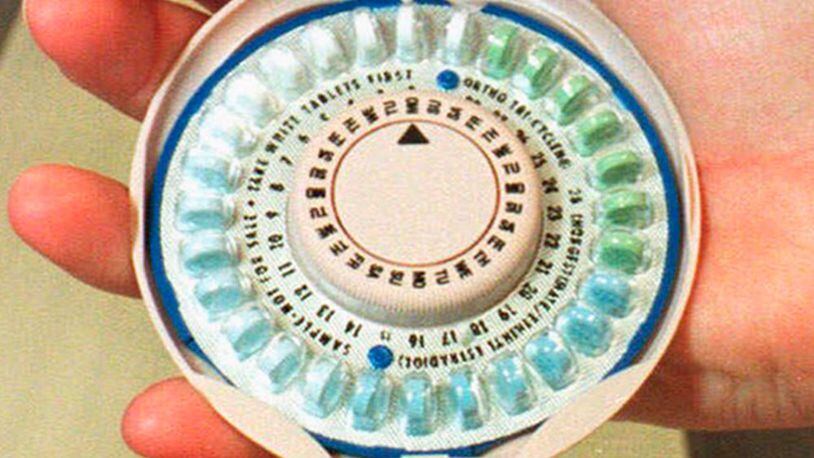
The suit accuses Qualitest, Inc., a subsidiary of Endo Pharmaceuticals Inc., of issuing birth control pill packages in which the pills were rotated, allowing the women to mistakenly take the placebo sugar pill, instead of the birth control pills, at a time of the month when they could become pregnant.
The recall affects these products: Cyclafem 7/7/7, Cyclafem 1/35, Emoquette, Gildess FE 1.5/30, Gildess FE 1/20, Orsythia, Previfem and Tri-Previfem, according to CNN.
Customers can call 1-877-300-6153 between 8 a.m. and 5 p.m. CT to get their questions answered, arrange to return their pills or report problems. Information is also available at http://www.qualitestrx.com/pdf/OCRecall.pdf.
About the Author